Category: Digital Health Law
-
Accounting for EU external effects: from clinical trials to data colonialism to AI ethics dumping

Against a backdrop of rapidly expanding health artificial intelligence (AI) development, this paper examines how the European Union’s (EU) stringent digital regulations may incentivise the outsourcing of personal health data collection to low- and middle-income countries (LMICs), fuelling a new form of AI ethics dumping. Drawing on parallels with the…
-
Artificial Intelligence in Radiology: Safeguarding Patients’ Rights in the Digital Era

Please cite as: Hannah van Kolfschooten, ‘Artificial Intelligence in Radiology: Safeguarding Patients’ Rights in the Digital Era’, European Radiology 2026. Artificial intelligence (AI) is now firmly embedded in radiology practice. From automated abnormality detection on chest radiographs to workflow optimisation in triage, AI is increasingly shaping diagnostic processes. Its promise…
-
Legal, ethical, and policy challenges of artificial intelligence translation tools in healthcare

Artificial intelligence (AI) translation tools, such as Google Translate and ChatGPT, are increasingly used in healthcare for medical communication to overcome language barriers between patients and providers. While these tools offer accessible and efficient translation, their use raises significant legal, ethical, and policy concerns. Key patients’ rights, including the rights…
-
AI Medical Devices after the Health Package: Innovation or lack of safeguards for patients?

As someone who has followed the AI Act closely from the very beginning, particularly in the context of healthcare, the European Commission’s new health package immediately raised some questions. Concretely, the Commission proposes to move the Medical Devices Regulation (MDR) and In Vitro Diagnostic Medical Devices Regulation (IVDR) from Section…
-
Artificial intelligence, intellectual property, and human rights: mapping the legal landscape in European health systems

Intellectual property (IP) rights and IP-related rights, such as trade secrets and regulatory exclusivities, play a crucial role in the development and deployment of artificial intelligence (AI) technologies. However, possible interactions may be anticipated when comparing the legal relationships formed by these rights with those established by human rights. This…
-
AI Chatbots for Promoting Healthy Habits: Legal, Ethical, and Societal Considerations
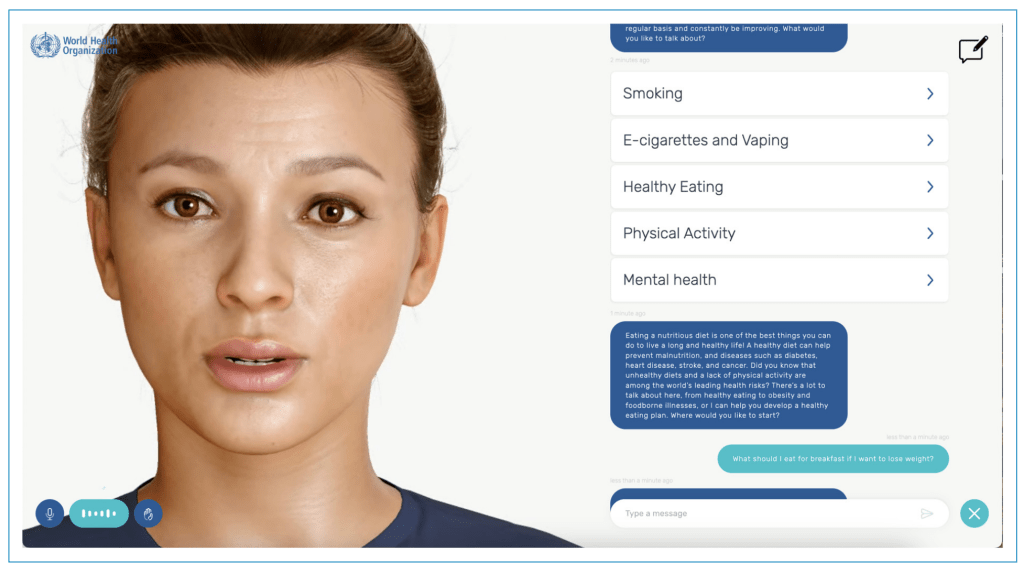
Machine learning-based artificial intelligence (AI) chatbots are increasingly used to promote health and encourage individuals to adopt healthier behaviors. Chatbots driven by generative AI (genAI) simulate human interactions through text or voice to generate personalized content with guidance on topics such as smoking cessation, nutrition, managing stress, and sleep improvement.…
-
Invisible prescribers: the risks of Google’s AI summaries
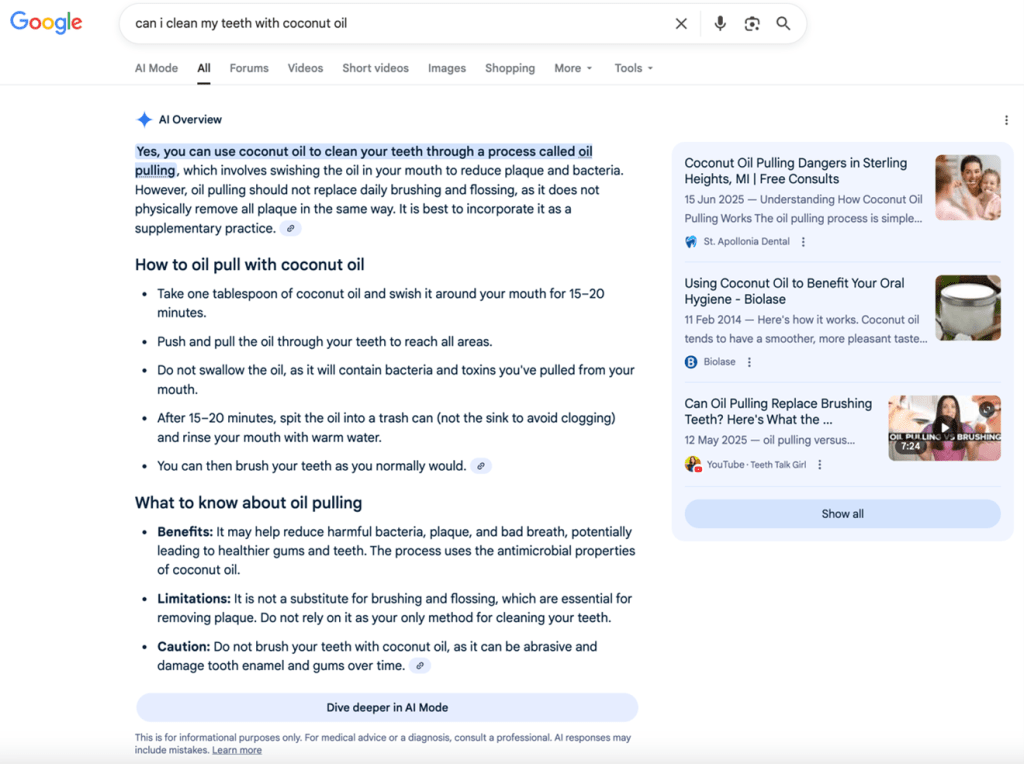
With digital technologies, your patients have a ‘doctor in their pocket’. But something new is happening when they search online for medical advice. Typing a question such as “Can I take ibuprofen with blood pressure tablets?” or “What helps against chest pain?” into Google no longer produces the familiar list…
-
WHO/Europe launches Technical Advisory Group on Artificial Intelligence for Health

WHO/Europe has formed the Technical Advisory Group on Artificial Intelligence for Health (TAG-AI) to guide the ethical, responsible and equitable use of AI in health across the WHO European Region. The group will serve as an advisory body to the WHO Regional Director for Europe for an initial period of…
-
Addictive Algorithms and the Digital Fairness Act: A New Chapter in EU Public Health Policy?

Picture a typical teenager waking up. Before even getting out of bed, they’ve already scrolled through TikTok, checked Instagram, and responded to Snapchat notifications. Each swipe delivers content fine-tuned by algorithms designed to maximize attention and engagement. Autoplay keeps the feed going. Notifications prompt more interaction. Autoplay keeps content flowing.…
-
Period Tracking Apps and Consumer Protections
Last week, reports emerged in the UK of concerned public health experts calling for public alternatives to commercial period tracking apps. Their research shows that women’s personal data is at great risk. More and more people who menstruate make use of period tracking apps, also referred to as ‘cycle tracking apps’ (CTAs).…
-
Global Health in the Age of AI: Charting a Course for Ethical Implementation and Societal Benefit

Artificial Intelligence (AI) presents unprecedented opportunities to transform healthcare worldwide, from improving diagnostic accuracy to expanding access in underserved regions. Despite this potential and growing investment, a significant gap persists between AI’s theoretical promise and its realised benefits in healthcare settings. This article examines the complex barriers impeding AI benefits…
-
Commentary: Why the EU AI Act Falls Short on Preserving What Matters in Health
Nicole Gross, Hannah van Kolfschooten & Alice Beck Response to BMJ 2025;388:r27 Dear Editor, In response to the BMJ article titled ‘AI in medicine: preparing for the future while preserving what matters’, we agree that an AI-driven future in healthcare is inevitable, and we must be prepared. The article emphasizes AI’s…
-
Commentary: Chatbots in medicine: certification process and applied use case

The article by Nehme et al. [1] provides a comprehensive analysis of the regulatory and certification challenges faced by healthcare chatbots. Using the confIAnce chatbot as a case study, the authors explore its classification as a non-medical device under the EU Medical Device Regulation (MDR) and the Swiss Medical Devices Ordinance (MedDO).…
-
Mental Health Europe report: Artificial Intelligence in mental healthcare

This study explores the opportunities, risks, and ethical considerations surrounding the use of artificial intelligence (AI) systems in mental healthcare and provides recommendations for their responsible implementation and regulation. Healthcare forms one of the most popular sectors for AI deployment in the EU. In mental healthcare, AI systems are used…
-
Towards an EU Charter of Digital Patients’ Rights in the Age of Artificial Intelligence

Abstract The rapid advancement of digital health innovation, including Artificial Intelligence (AI), is transforming healthcare. The growing role the European Union (EU) plays in regulating the use of AI in healthcare renders national laws insufficient to safeguard patients from unique AI-related risks. This underscores the urgent need for the recognition…
-
When people become data points – the potential impact of AI in mental healthcare

Hannah van Kolfschooten & Janneke van Oirschot Introduction Artificial intelligence (AI) holds great promise for transforming mental healthcare. From personalized treatment plans to early detection of mental health conditions, AI could make mental health services more accessible and effective. AI systems are already being developed for various applications, including diagnostic…
